In today’s FSMA-focused world, performance validation is key. A food processor can put into place a sophisticated, well researched sanitation protocol or system, but without proper validation, what good is the system? How does one know the system is performing as intended? A warm, fuzz feeling is not enough. These operations require methods and means of validation for every aspect of its food safety and sanitation program. This applies to cleaning procedures, equipment maintenance, SSOPs, and overall hygienic process and facility design.
Your Concrete Floors and Walls Are Often Overlooked
The truth is your floors and walls should be a major focus in your food processing operation. Concrete floors and walls, when untreated, can provide the perfect environment for unwanted dangerous microbe growth. Concrete is a porous material that retains and transmits moisture, making it difficult to properly clean and sanitize.
The FDA has recommended guidelines when it comes to floor and wall coatings, but these guidelines are relatively vague. The FDA recommends that the floor must be designed to be easy to clean and maintain and to protect the product from microbial, physical and chemical contamination. For example, designing food contact surfaces to be smooth, nonabsorbent, smoothly bonded, without niches, and sealed would make these surfaces easier to clean and thus, would prevent the harborage of microbial pathogens.
In the past, it was not possible to validate your facility floor and wall performance. Concrete floor and wall coatings were merely viewed as a “necessary evil”. However, in recent FSMA-focused times, the need for validation is of the utmost importance.
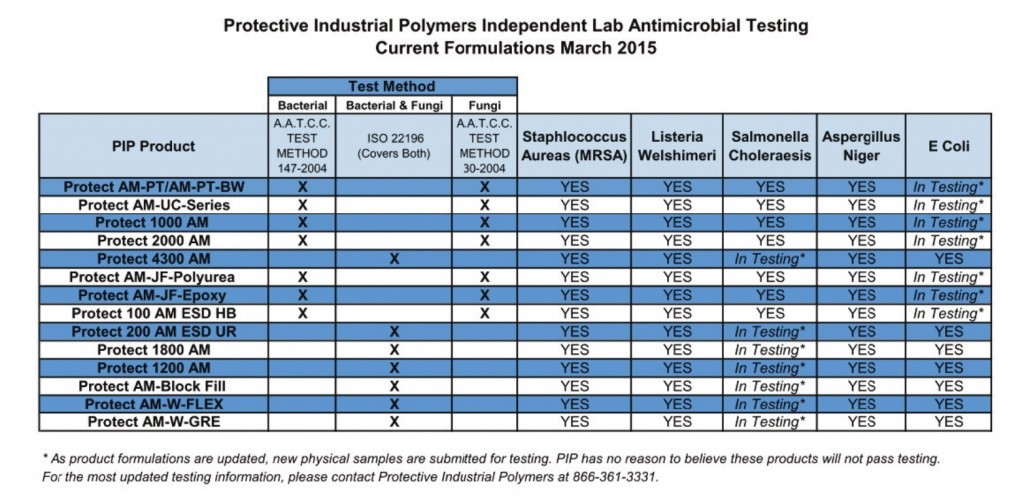
There are, however, floor and wall coating systems available today that bridge the gap from the “whitewash mentality” of the past and the modern requirement of redundant, validated antimicrobial protection. Protective Industrial Polymers has coined the term “Validated Performance Technology”, as it relates to their line of antimicrobial floor and wall coating systems.
PIP’s InhibiCrobe Floor and Wall Systems have been independently microbiologically-tested to ensure the cured coating(s) perform as designed. This means these systems will not support the growth of dangerous microbes, such as Listeria and Salmonella, among others. Each product utilized in every InhibiCrobe system has been independently tested to validate its performance characteristics.
Now, one can have the best of both worlds: Validated Performance Technology and a warm, fuzzy feeling.
Want to learn more about treating your processing floors with redundant, validated antimicrobial protection? Download our latest whitepaper “Antimicrobial Polymer Floor & Wall Systems: Validated Performance Technology”.